Multiple brands of lubricant eye ointments are being recalled due to a risk of infection after federal inspectors found unsterile conditions at the Indian plant where the products were manufactured.
The recall by Brassica Pharma Pvt. in Thane, a city in the Indian state of Maharashtra, comes after a deadly outbreak last year of eye infections linked to artificial tears made by another Indian firm.
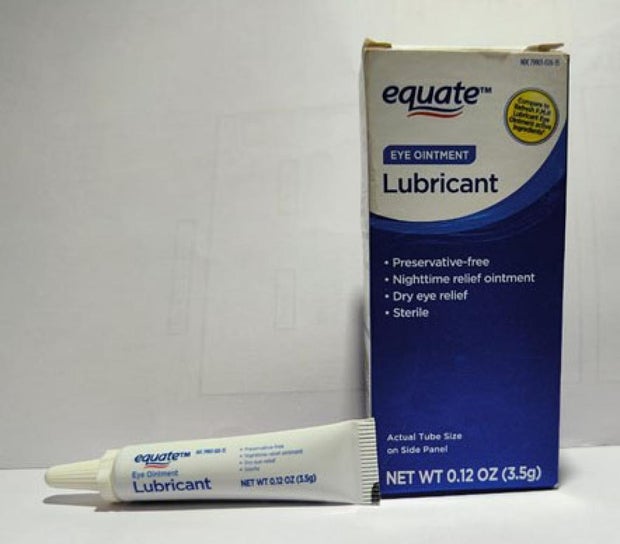
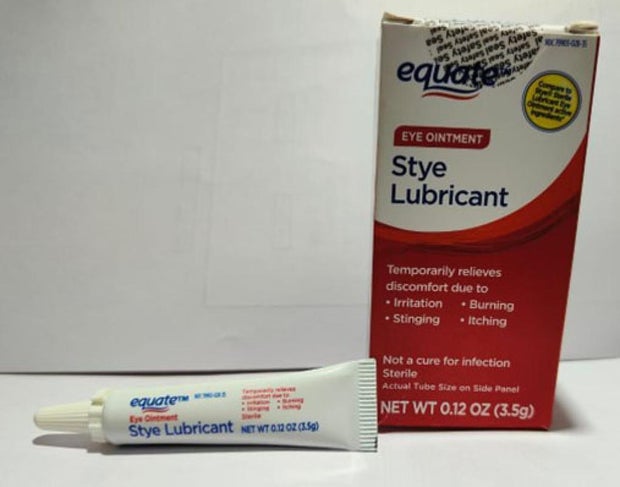
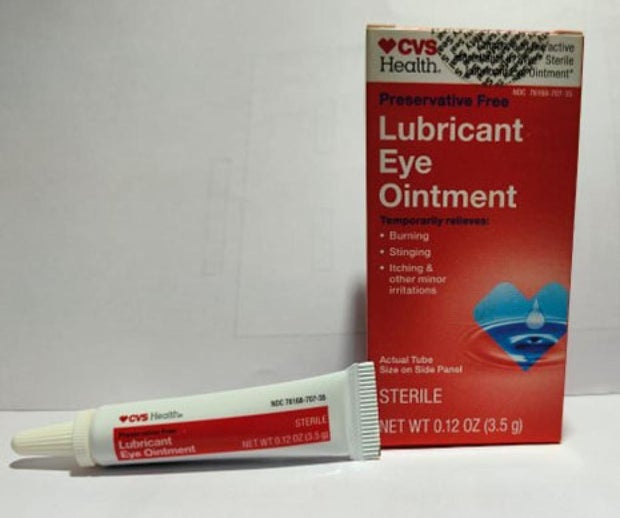
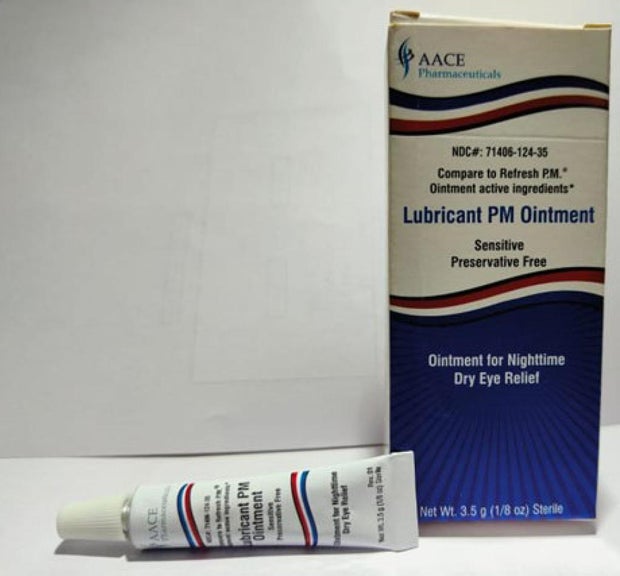
Sold nationwide by retailers including CVS Health and Walmart, the latest recall involves four products by brands Equate, CVS Health and AACE Pharmaceuticals, according to the notice posted Monday by the U.S. Food and Drug Administration.
All have expiration dates starting April 2024 and running through September 2025. The recalled products include:
- Equate Lubricant Eye Ointment in a 3.5-gram tube, packaged in box with UPC code: 681131395298.
- Equate Style Lubricant Eye Ointment in a 3.5-gram tube in box with UPC code: 681131395304.
- CVS Health Lubricant Eye Ointment in a 3.5-gram tube in box with UPC code: 050428634141.
- Lubricant PM Ointment in a 3.5 gram tube, sold in box with UPC code: 371406124356.
U.S. Food and Drug Administration
U.S. Food and Drug Administration
U.S. Food and Drug Administration
U.S. Food and Drug Administration
People should stop using the recalled eye ointments and can return them to the place of purchase. Those with questions can call 1 833-225-9564 or info@brassicapharma.com.
EzriCare artificial tears were among the brands recalled last year by Delsam Pharma of Chennai, India, as health officials identified 81 people in 18 states as suffering from infections, with four deaths and multiple cases of vision loss reported.
In January, the FDA warned against copycat eye drops because of the risk of infection.
Thanks for reading CBS NEWS.
Create your free account or log in
for more features.